Introductory note. When I started work on this piece I was primarily interested in how Pfizer misrepresented side effect profiles in its trials. The more I looked into this topic the more concerned I became about the novel trial architectures being employed in Randomized Control Trials. I have retained my starting point for the historical record but have expanded the scope of this investigation to include my serious misgivings regarding the globally distributed and outsourced business model and all that entails.
Pfizer Misleads on Vaccine Safety
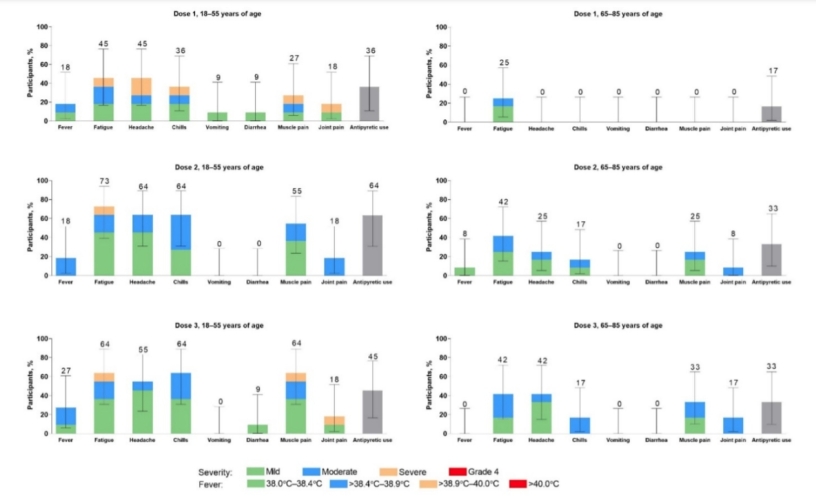
Behind the usual claims of over 90% efficacy for booster shots, in the appendix supporting their press release, Pfizer gives us some disturbing figures related to adverse events across all 3 doses, particularly in the under 55 cohort grouped to the left of the charts. Bear in mind, that as I will show later this handful of side-effects were pre-approved by Pfizer and bullet listed as the only adverse events for trial participants to register using the trial app/electronic diary. Papers since released by the FDA show that there are many more.

This is deeply disturbing in that Pfizer in their original effective press release for their vaccine claimed that
A review of unblinded reactogenicity data from the final analysis which consisted of a randomized subset of at least 8,000 participants 18 years and older in the phase 2/3 study demonstrates that the vaccine was well tolerated, with most solicited adverse events resolving shortly after vaccination. The only Grade 3 (severe) solicited adverse events greater than or equal to 2% in frequency after the first or second dose was fatigue at 3.8% and headache at 2.0% following dose 2. Consistent with earlier shared results, older adults tended to report fewer and milder solicited adverse events following vaccination.
https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine
Pfizer claimed in October 2020 that only 3.8% of participants suffered fatigue as a grade 3 adverse event and only 2% suffered headaches. Grade 3 events are shown in the chart in the orange bars.
In the under 55s roughly 10% suffered severe fatigue, 20% severe headaches, 10% severe joint pain and 10% severe muscle pain after dose 1. In the appendix, Pfizer defines severe events as “prevents daily routine activity” for example time off work. It seems pretty clear from this that Pfizer completely concealed the fact that in the under 55s, who are least at risk from Covid: that the vaccine was not well tolerated at all given that at least 20% of participants were unable to carry out daily routine activity or go to work. In this particular age group this is similar to the actual effects of catching Covid. Given that vaccines provide highly questionable protection, vaccinating this group is effectively giving them Covid twice.
At a data lock date of 10th November 2021, for Pfizer across both doses, only 24,362 events of headaches and 20,312 events of fatigue had been reported to the UK yellow card surveillance system from 24.2 million first doses. Given the high rates of moderate and severe adverse events reported in Pfizer’s charts there are clearly millions and millions of events missing from the yellow card so called surveillance ‘safety’ system.
Pfizer’s software and trial protocol was designed to only allow users to report this very small subset of adverse events and systematically excluded the reporting of any other of the dozens of different types of events that have been subsequently reported to the VAERs and Yellow Card systems in the USA and UK respectively. It should also be noted that Pfizer restricted the collection of any AE data to just 7 days after each shot so there was no attempt whatsoever to record any longer term events.
This small subset of events, which are largely derived from symptoms of Covid-19 itself as might logically be expected from artificially infecting the body with Sars-Cov2 spike proteins, together with effects such as pain and swelling at the site of injection: were the only events that Pfizer anticipated and which they would ever acknowledge as being causally related to vaccination. Any events outside of this tiny subset were not possible to report via the TrialMax smart phone application and system which Pfizer employed to ‘record’ safety data nor were they acknowledged if recipients contacted their local trial centre with events which did not fit the preconceived definition of ‘acceptable’ events according to the proposed safety reporting protocols.
How to design software to conceal adverse events
In early November 2021, Senator Ron Johnson hosted an expert panel on vaccine mandates, efficacy and safety at which two participants of vaccine trials were present. Stephanie and daughter Maddie de Garay who was part of the Pfizer children’s trial and Brianne Dressen who was a participant in the US AstraZeneca trial for adults. Maddie is still currently in a wheelchair and needs assistance feeding and breathing.
The entire video is a must watch as it shockingly illustrates the morally disgusting inhumanity which the vaccine injured are systematically being subjected to in the USA. This is nothing short of a thoroughly organized atrocity, perpetrated by sociopaths who are entirely devoid of human sympathy or empathy. It beggars belief that out of the entire US congress there is but a single senator who has the most basic humanity and empathy to seek to help these vaccine victims and he is a republican for god’s sake! If anyone needed proof that our political systems and parties have become sociopathic entities that have no regard for the wealth, health and safety of the general public then this is it.
Those despicable elements aside, the information that is relevant to this discussion can be found if you start at 2 hours 28 minutes into the proceedings. I have transcribed the relevant testimony below. First off we find Maddie’s mother confirming the 1st dose results shown in the Pfizer charts.
When she got her first dose her reaction was typical, she had fever, body aches and fatigue and it went away in a couple of days….when she got her second dose she had immediate pain…in less than 12 hours she developed severe abdominal pain, horrible nausea, painful electric shocks in her spine and neck, her hands were ice cold when you touched them and on her feet. Pain all over her body. Her vaccine arm went numb….she had chest pain, tachycardia that was seen on a EKG…she was extremely dizzy, she felt like she couldn’t stand up.
I went in trusting the drug companies, the FDA, the CDC, the hospital where the trial was held…So when you enter the trial everybody uses a trial app…its called TrialMax and they log the reaction for 7 days after each dose – that’s it. The app only allows you to record for solicited adverse events like fever, redness, injection site pain, swelling, headache, vomiting and other typical expected reactions. That is it and you say mild, moderate and severe. Severe means you had to go to the ER. You record your fever like the actual amount. There’s no free form at all to fill in any other reaction that you have beyond the typical non serious events other than anaphylaxis. What you have to do if you have any other adverse event is you have to call the study Doctor or principle investigator and there’s no way of entering any unbiased way of documenting it... So we did what we were told and called the study Doctor and they told us to go to Cincinnati Children’s ER where the trial was held to check for appendicitis….What made it into the trial record is unclear and yes we did ask several times and have it documented, we still don’t know what was actually recorded…..in the EUA amendment Maddie’s reaction was reduced to 5 lines which was eventually diagnosed as stomach ache…by the data cut off for the trial Maddie experienced over 35 adverse events…she went to ER 9 times and was hospitalized 3 times for a total of 63 days. They did everything in their power to hide what happened to her.
Big Pharma gets to ‘approve’ which adverse events it will accept
The gathering of safety data in the vaccine trials took place against a set of adverse events approved or authorised by the manufacturers themselves. The entire context of possible side-effects was entirely governed and strictly limited by the restriction of around 14 adverse events the manufacturers were prepared to causally accept which were then enforced by the design choices of the software itself.
Brianne Dressen confirms the same modus operandi in the AstraZeneca trials.
Like Maddie’s trial we had a tracking app. Like Maddie’s trial we had pre-designated symptoms in a bullet list with no free form to enter other symptoms.
The fact that exactly the same set of ‘approved’ side-effects and data entry limitations were imposed in the AstraZeneca trials suggests that this was a tactic which had been globally shared and seized upon in order to hide all other side effects outside of those ‘authorized’ by the manufacturers. Brianne Dressen had a very severe reaction to the first dose and AZ simply removed her from the trial but reported this as her dropping out of the trial on her own volition. Her adverse reactions were effectively disappeared from the trial. This is just out and out fraud as the wilful concealing of adverse events which did not fit their narratives.
Did Pfizer mislead participants as to event grading?
In her testimony regarding event grading of mild, moderate and severe, Stephanie de Garay said “ That is it and you say mild, moderate and severe. Severe means you had to go to the ER“. This is actually incorrect. Grade 3 severe problems were defined in the protocol as anything that prevented one from following a normal daily routine or go to work etc. Grade 4 events were those cases where a participant attended ER or medical centre. You can see in the PDF below, that no grade 4 events appear in the charts for Maddie’s trial despite her having attended ER 9 times and spending 63 days in hospital.
https://www.nejm.org/doi/suppl/10.1056/NEJMoa2107456/suppl_file/nejmoa2107456_appendix.pdf
Stephanie seems to be a reasonably intelligent lady so it would be reasonable to enquire as to how this misunderstanding came about and whether Pfizer misled participants to only record an event as severe if it involved a visit to the Doctor or ER. If this is the case then the net effect of this would be to downgrade all Grade 4 events to Grade 3 events and all Grade 3 events to Grade 2 events. If we apply these considerations to our interpretation of Pfizer’s charts then many of the Grade 3 events become Grade 4 events and all the moderate events become severe events.
It gets even worse
The vaccines manufacturers assured trial participants that they would pay for any medical care bills arising from any severe adverse reactions from vaccination. The was an empty promise in that they refused to acknowledge any severe reactions outside of their authorized list. This left participants with severe reactions like Brianne Dressen having to refinance their homes in order to cover the cost of their medical care. Of course its not just Pfizer that are denying adverse events outside of their authorised bulleted list, the FDA and CDC have also been applying this approach.
This is an obvious cover up because we find that in October 2020 the FDA had a working list of the possible adverse events they were anticipating and many of the events they are now refuting were on that list. [This slide was shown in error and only for around 1/20th of a second – the link is below but you better be quick on the pause button!]

https://youtu.be/1XTiL9rUpkg?t=9220
So on October 22nd 2020 the FDA already had a working list of severe adverse events on their watch list. Many of these are events have been reported to VAERS and the FDA has taken to denying them to be vaccine related and accounting for vaccine induced mortality as being ‘background’ noise. Charts from VAERS data show this cannot be the case.

The blue line in the chart above plots what level of background deaths is to be expected. This chart shows that excess mortality in relation to dosing date remains elevated for up to 21 days from date of dose. No explanation other than vaccine induced mortality can be deduced from this chart.
Faking Vaccine Efficacy Using Important Protocol Deviations
Exclusions from the evaluable efficacy population occurred for 5.1%(78) of the BNT162b2 group…due to receipt of Dose 2 outside the protocol defined window of 19-42 days after Dose 1…or due to other important protocol deviations on or prior to 7 days after Dose 2…primarily related to vaccine thawing, dilution, and/or administration issues that are not applicable to placebo.
https://www.fda.gov/media/153409/download
IPDs will likely occur on a far larger scale in the real world than would be the case in a strictly managed RCT. The very notion of whether a protocol deviation was ‘important’ enough to justify exclusion is also open to question. Every Pfizer vaccination in the UK would have to be removed from evaluable efficacy as the 2nd dose was administered at least 3 months after the first. There would be a huge increase in issues with vaccine thawing, dilution and administration as many thousands of people needed to be trained to vaccinate efficiently and there was no monitoring or oversight of how effective these training and procedural processes might have been: particularly when it involved such unusually low temperature storage and related handling difficulties. This trial only involved 1450 participants yet Pfizer’s poor quality controls on even such a small scale trial, led to a 5.1% failure rate. This is hardly clinical or scientific excellence on display here. Real world failings could be considerably higher though there is no quality control monitoring taking place to verify any of this.
If we are to accurately assess the efficacy of any given treatment which is destined to be deployed and used in the real and thoroughly imperfect world in billions of doses, then we need an evaluation to take place that takes all of these unavoidable accidents, mistakes and imperfections into its consideration. This means that it is completely and utterly inappropriate to remove such data in order to present a case of how efficacious a vaccine might be within an artificially contrived model of a perfect world. Pfizer removed a total of 78 participants from the treatment arm of its trial. In the placebo arm, 16 of the 751 subjects were diagnosed with Covid-19 after dose 2. That gives us an infection rate of 2.1% or one in 47. This means that of those 78 participants removed from the trial on the grounds that they were ‘technically’ unvaccinated, then we would expect a maximum of 2 to have tested positive with Covid-19. Pfizer need to disclose their data as to how many of these 78 excluded participants tested positive. Anything over 3 would suggest that Pfizer is making an assumption of problems with thawing, dilution or out of range dosing in order to account for the lack of vaccine efficacy in those 78 participants but was making such an assumption without any real evidence. I’m willing to take a guess that at least 10 of those 78 subjects had a positive PCR result. We will probably never know and I’ll explain why later.
Unadulterated Data Show Vaccines Don’t Work
In my last article I showed how the very best quality data which excluded false positives by only using data from PCR tests with very low cycle thresholds proved that those vaccinated had the same levels of virus as the unvaccinated and that the vaccines do not reduce disease severity in the under 50s as there was no difference in terms of infection fatality rates which were 0.05% unvaccinated against 0.06% fully vaccinated. The PHE data also confirmed that the fully vaccinated were catching Delta from at least mid February and that viral loads between the fully vaccinated and the unvaccinated by June were just 0.2 of a PCR cycle threshold.
The elephant in the room then becomes the question as to why is it, that if vaccine efficacy in the real world is ZERO – then how was it that the RCTs all showed 95% efficacy. The only explanation is that the RCTs were fraudulent.
In an excellent presentation, the Canadian Covid Care Alliance show that Pfizer failed to test no less than 3,410 participants in the EUA winning RCT were symptomatic of Covid-19 but were never tested.
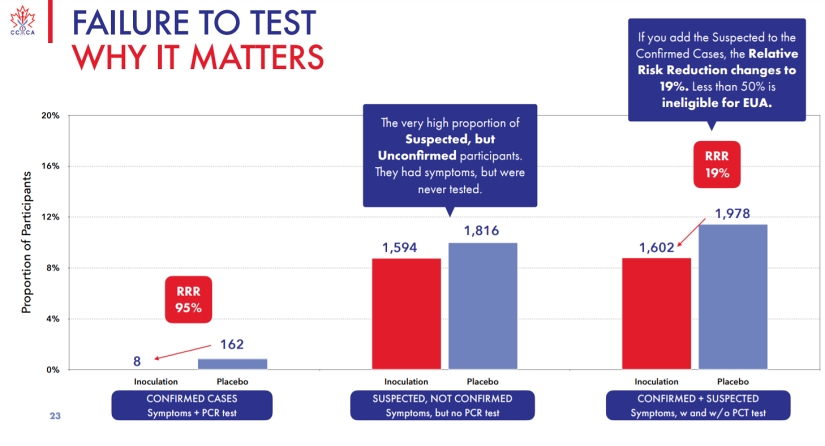
The Boulware PEP RCT study of HCQ used a remote diagnosis of symptomatic participants in order to discredit HCQ, so if such techniques are credible under accepted gold standards for RCT design then all of these 3,410 participants could have been assumed to be Covid-19 positive, in which case as the CCCA point out: the relative risk reduction fall to 19% which was well below the standard required to obtain an Emergency Use Authorization.
Unblinding Trials By Side Effect Analysis
Even this small 19% RRR is questionable in the Pfizer could logically deduce which trial participants had received the vaccine by analysing side effect profiles. In published data for later trials we see that at 30mcg dosage: Fever, Chills, Muscle Pain and Joint Pain were significantly more common in the vaccinated group with Fatigue and Headaches not far behind.

Fever was 43X more common in the vaccinated group, chills 9X more common etc.
Even if you can only statistically unblind 60% of vaccine recipients that can be enough to swing the trial into a significant result in your favour by strictly limiting or eliminating testing in the now unblinded group. No wonder the FDA and Pfizer do not want to publish their underlying data and records.
What follows is my assessment of Pfizer’s initial trial from a bigger picture perspective of the side effects of the continued drift toward outsourcing functionality in government and public health to the private sector.
Outsourcing Induced Irresponsibility and Unaccountability
It is disturbing when one sees the amount of outsourcing of traditional public sector functionality to that of the private sector. From the start of the financial crisis in 2007/8 politicians took no responsibility to address any solution to the problems, they abdicated from their power and outsourced the solutions to central bankers who have not stopped printing money since. Central bankers can only notionally regarded as public employees as they retain myriad ties and relationships with the most powerful banks and financial institutions whose interests they really represent.
We see exactly the same with Covid-19. Politicians abdicated from their power and outsourced the solutions to Bill Gates, Big Pharma and a highly select band of ‘The Science’ scientists. On both occasions they effectively outsourced responsibility and accountability, sacrificing democracy in the process. What we will end up with when any Covid-19 enquiry worthy of the name takes place is that accountability and responsibility will be endlessly displaced and dispersed among the many different bodies operating behind the scenes. In effect responsibility and accountability cease to exist. Worryingly that’s more likely to be an intended effect rather than some kind of accidental side effect of outsourcing.
One of the core problems of outsourcing functionality is that in the event of a policy failure or fraud then any audit or enquiry into the issues is confronted by multiple parties involved in attempting to shift blame onto each other making it hard to determine who is ‘responsible’ or ‘accountable’. This is a major problem now in Western neoliberal societies outsourcing government and NHS functionality to a not entirely trustworthy, efficient or reliable private sector who freed by neoliberal deregulation from policing, scrutiny and audit: then by only being charged with a neoliberal duty to maximize profits and returns to shareholders, it should be no surprise that they have morally and ethically degenerated into bad actors within a general systematic drift to corruption that has slowly developed since 1980. Since the GFC this drift to corruption has only accelerated.
Government, policy execution, responsibility and accountability can only take place when a given or designated party takes full ownership of the policy or function. A clear example of this is ownership of the function of reporting vaccine adverse effects. No-one in either the USA or the UK has been directly given ownership of this task and functionality. It has ineffectively been virtually ‘outsourced’ or left as a free floating ‘responsibility’ between a number of different entities most of whom do not have the time, skills or inclinations to complete the task. Consequently for the most part it is simply not happening. When you are rolling out an experimental vaccine to billions of people across the world, the failure to assign ownership of this functionality is probably the biggest ‘mistake’ or intentional cover-up in human history. Can you imagine any business could function effectively if no-one was given the ownership for accounting functions such as issuing invoices or paying wages and such tasks were left free floating in the hope that someone somewhere in the organization might happen to occasionally fulfil the function but only in the right circumstances?
It seems patently obvious that those agents injecting patients should have been assigned ownership of the safety reporting function as they had direct contact with patients and open communications. They would then be tasked to make follow up phone calls at 3 days, 7 days, I month, 3 months, 1 year and 3 years. All adverse events would be required to be reported regardless of any causal attribution to vaccination and all patients with serious events should have medical examinations. Such reliable functionality could not be achieved using an NHS app, Yellow Card or VAERS systems as by design they allow patients themselves and 3rd parties to enter what can later be deemed to be so called untrustworthy, anecdotal and malicious false events. It is this built in feature of those systems that render them not fit for purpose as they are designed to fail in that as soon as they signal safety issues with any treatment, then all those events can be dismissed as an effect of built in flaws in the system design to begin with. Our medication safety surveillance systems were designed to be inherently discreditable.
Disturbing Outsourcing Evolutions in RCT Design
RCTs have been considered (wrongly in my mind) to be the gold standard of evidence for evaluating medical treatment efficacy. One of my concerns that I covered in 2020, is that they are very expensive to carry out and are biased against multi-agent repurposed treatments interacting in synergy as they are functionally designed to favour novel single agent, magic bullet treatments. No wonder Big Pharma and ‘The Science’ is so keen on them. Now we can accept that RCTs are useful in some circumstances but only if they take place transparently with excellent training and uniform application of standards and protocols along with rigorous management and oversight for the most rigorous quality control. All data should be made available for public scrutiny. This data availability has to be made well in advance of any licensing or EUA.
Before many of us started looking more closely at RCTs when we suspected that a lot of suppression of early treatments, suppression of adverse events and a general sexing up of vaccine efficacy was taking place, I think many of us previously had a heavily idealized view of the integrity and efficiency of how what we assumed to be very strictly managed RCTs were carried out in the real world.
Since Covid-19 we now find that there is a new and very expensive gold standard in RCTs where RCTs are now required to be conducted in dozens and even hundreds of trial ‘sites’ which are globally distributed around the world in order to apparently show global efficacy and this is true not just of vaccine medications but also novel treatments such as Merck’s Molnupiravir. So we find in Pfizer’s orginal RCT which landed it an EUA:
The Phase 3 clinical trial of BNT162b2 began on July 27 and has enrolled 43,661 participants to date, 41,135 of whom have received a second dose of the vaccine candidate as of November 13, 2020. Approximately 42% of global participants and 30% of U.S. participants have racially and ethnically diverse backgrounds, and 41% of global and 45% of U.S. participants are 56-85 years of age. A breakdown of the diversity of clinical trial participants can be found here from approximately 150 clinical trials sites in United States, Germany, Turkey, South Africa, Brazil and Argentina. The trial will continue to collect efficacy and safety data in participants for an additional two years.
https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine
An RCT carried out in over 150 trial sites distributed across 6 different countries with multiple languages and time zones will be an administrative ‘Nightmare on Elm Street’ to properly establish with adequate and consistent protocol training and assessment procedures. It is a massively overly complicated architecture that will be too difficult to manage, monitor and effectively oversee. Such an architecture was further incapacitated by having to be rushed out at warp speed. This is the worst possible architectural design for an RCT! Multinational companies might successfully operate such complicated business architectures but it takes them decades to establish and this cannot effectively be done in a few short months.
Worse still, Pfizer did not carry out trials at these 150 trial sites themselves, they outsourced the functionality, responsibility and accountability to contractors which notionally distances Pfizer from responsibility and accountability in relation to disputes around the contested efficacy of their results. Given the massively over complication in the underlying architecture and the rushed nature of its roll out we would expect that behind the scenes there would be serious issues that would be logically imposed on such trials. Sure enough, in addition to the issues I discussed above in relation to important protocol deviations, then thanks to a whistle blower at the contractor ‘Ventavia’ we get to see how these predictable effects necessarily became manifest.
for researchers who were testing Pfizer’s vaccine at several sites in Texas during that autumn, speed may have come at the cost of data integrity and patient safety. A regional director who was employed at the research organisation Ventavia Research Group has told The BMJ that the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer’s pivotal phase III trial. Staff who conducted quality control checks were overwhelmed by the volume of problems they were finding… Jackson has told The BMJ that, during the two weeks she was employed at Ventavia in September 2020, she repeatedly informed her superiors of poor laboratory management, patient safety concerns, and data integrity issues….In a recording of a meeting in late September2020 between Jackson and two directors a Ventavia executive can be heard explaining that the company wasn’t able to quantify the types and number of errors they were finding when examining the trial paperwork for quality control. “In my mind, it’s something new every day,” a Ventavia executive says. “We know that it’s significant.”…Ventavia was not keeping up with data entry queries, shows an email sent by ICON, the contract research organisation with which Pfizer partnered on the trial. ICON reminded Ventavia in a September 2020 email: “The expectation for this study is that all queries are addressed within 24hrs.” ICON then highlighted over 100 outstanding queries older than three days in yellow. Examples included two individuals for which “Subject has reported with Severe symptoms/reactions … Per protocol, subjects experiencing Grade 3 local reactions should be contacted. Please confirm if an UNPLANNED CONTACT was made and update the corresponding form as appropriate.”.
In several cases Ventavia lacked enough employees to swab all trial participants who reported covid-like symptoms, to test for infection. Laboratory confirmed symptomatic covid-19 was the trial’s primary endpoint, the employee noted. (An FDA review memorandum released in August this year states that across the full trial swabs were not taken from 477 people with suspected cases of symptomatic covid-19.)
“I don’t think it was good clean data,” the employee said of the data Ventavia generated for the Pfizer trial. “It’s a crazy mess.”
A second employee also described an environment at Ventavia unlike any she had experienced in her 20 years doing research. She told The BMJ that, shortly after Ventavia fired Jackson, Pfizer was notified of problems at Ventavia with the vaccine trial and that an audit took place.
Since Jackson reported problems with Ventavia to the FDA in September 2020, Pfizer has hired Ventavia as a research subcontractor on four other vaccine clinical trials (covid-19 vaccine in children and young adults, pregnant women, and a booster dose, as well an RSV vaccine trial;
https://www.bmj.com/content/375/bmj.n2635
Given the massively over complicated trial architecture all these failings were guaranteed to occur. Ventavia would not in any way have been unusual in this respect. A number of issues come out of this. Not least is that it appears that Pfizer also outsourced the functionality of contractor oversight itself to yet another third party entity: ICON.
So where does the real responsibility and accountability actually lie between all these parties? We don’t know if Pfizer instructed contractors to mislead on event grading or whether it was the contractors themselves or whether it was a simple misunderstanding on the part of one person explaining event grading. Real world data continually show that Pfizer’s claims of 95% efficacy was completely false and that the trials were fraudulent even if that fraud may have been conducted by the contractors. You cannot have a vaccine that has no efficacy in the real world and only works in RCTs without concluding that there was something seriously wrong with the trials and this should be a matter for urgent investigation given what is at stake in all of this.
Why was Archaic Pen and Paper Technology Adopted by Ventavia?
In our high tech modern era, then it comes a something of a surprise that a company backed by billions of government dollars in a global trial of such unrealistically ambitious proportions could not even provide its contractors with standardized RCT software which could have helped consistently manage and process data directly linked to Pfizer’s central databases! As a company Ventavia does clinical trials all the time: why didn’t it possess software for processing? In any so called ‘gold standard’ trial, this would have been a minimal requirement in terms of quality control as those are the kind of things that computerized systems do really well if they are correctly designed. Many of such trial protocols and requirements can be standardized and built into a well designed systems with automated process flows so as to largely mitigate the possibility of anyone getting things wrong. You can never eliminate human error but well designed RCT and safety monitoring systems would minimize the effect of human error and common failures in personal and paper based work flows and management. It seems that all aspects of these trials were conducted on pen and paper forms which at some time would have to be hand typed into whatever data gathering systems Pfizer was using. This in itself leaves much room for paperwork going astray and common data entry mistakes.
This was clearly not any kind of gold standard but something more akin to a badly managed chaos, that was always to be expected. This does not happen by accident it is a direct effect of the architectural choices made with regard to the trial infrastructure and the modern business practice of outsourcing core business or functional requirements to black-box, incompetent third parties without any effective training, management and monitoring protocols being established. Pfizer should never have notionally conducted these trials in the first place as they should have been done by public bodies who could take complete ownership, responsibility and accountability over all aspects of such trials which should only be carried out in good faith by publically funded hospitals who have no financial conflicts of interest. Pfizer’s gold standard RCTs are in fact an architectural standardization or embedding of fraudulent behaviours and systematized incompetence which have become the endemic hallmarks of Western neoliberal economies across all corporate industries.
Outsourcing: Contractual Financial Dependency and Built-in Conflicts of Interests
One of the main problems that arise once one adopts the business model of outsourcing core functionality to obscurantist third parties is that there are unstated symbiotic relationships constitutive of systemic bias and conflicts of interest which are generated by the new business architectural infrastructure itself. In order to highlight these issues I would refer to a small portion of the BMJs whistle blower article whose significance likely went un-noticed.
A second employee also described an environment at Ventavia unlike any she had experienced in her 20 years doing research. She told The BMJ that, shortly after Ventavia fired Jackson, Pfizer was notified of problems at Ventavia with the vaccine trial and that an audit took place.
Since Jackson reported problems with Ventavia to the FDA in September 2020, Pfizer has hired Ventavia as a research subcontractor on four other vaccine clinical trials (covid-19 vaccine in children and young adults, pregnant women, and a booster dose, as well an RSV vaccine trial;
Now given what was at stake in all of this, I was totally surprised that given the atrocious failures of Ventavia to properly execute their functional requirements: that Pfizer saw fit to re-commission them for future trials. In this very difficult situation, if Pfizer had wished to project some kind of systemic integrity over the conducting of this functionality by remote 3rd party entities, then I think I would have been inclined to have sacked that particular firm from any future trials as their performance was completely unacceptable. Much like Watergate, once a system supposedly purges itself of the transgressor, then the rest of the system is automatically assumed to be functioning correctly or ethically, even if that is seldom the case. It is consequently very difficult to rationalize Pfizer’s response. In as much as they did not see such transgressions as worthy of exclusion from future trials, then this either means they are completely unconcerned about such shoddy practices amongst their contractors or it is evidence that there were other systemic reasons why Pfizer deduced that it was better to keep this company on board rather than take punitive actions in the circumstances which should have been appropriate and at least given the illusion that the trial as a whole had functioned properly. As it stands one can only conclude that such transgressions were equally distributed among the contractors as Pfizer failed to penalize this one company and this confirms my hypothesis concerning the unwieldy architectural infrastructure and hasty roll outs as leading to such generalized problems.
In teasing out these structurally guaranteed logical inconsistencies one can recognize that in an outsourced competitive market place it is clearly not in the interest of firms who are financially relying on repeat business from Big Pharma and who take on the functionality of ‘trial sites’: to continually output negative results across different trials as Pfizer would terminate their relationship with the company even if they did so because they viewed the contractor as being somehow ‘unlucky’ for them in comparison to other providers giving them good results. There is obviously always an incentive and conflict of ethical interests for the dependent contractor to please the pharmaceutical company that is paying them by producing results the pharmaceutical company is hoping for. Such conflicts of interests never need to be formally acknowledged as both parties will tacitly gravitate toward such conclusions and outcomes which are never formally expressed. Obviously, without a full audit and no such audit is ever likely to take place, then it is hard to assess how much this has a bias effect on RCTs. For myself at least it seems patently obvious and predictable.
It is the very structure of trials in which such key functions are outsourced to contractors that introduces this problematic in the first place and that outsourcing is an entirely inappropriate model to employ in RCTs.
Pfizer Trial Architecture Renders It Impossible To Audit
The FDA has been assigned with two functions regarding vaccines. The first and primary function is to promote vaccines and a secondary function is to monitor vaccine safety. These two functions are mutually incompatible. The FDA cannot report honestly on vaccine safety if that information would detrimentally impact on its ability to promote any given vaccine and vaccines in general. This dualism in its roll is further impacted by the fact that it receives large amounts of funding from Big Pharma and many officials within the FDA hold patents on vaccines themselves. So we find that the FDA commits very little in the way of resources to any proper auditing of vaccine RCTs. “The agency’s oversight capacity is severely under-resourced. If the FDA receives a complaint about a clinical trial, she says the agency rarely has the staff available to show up and inspect…..There’s just a complete lack of oversight of contract research organizations and independent clinical research facilities”
When it comes to the FDA and clinical trials, Elizabeth Woeckner, president of Citizens for Responsible Care and Research Incorporated (CIRCARE),3 says the agency’s oversight capacity is severely under-resourced. If the FDA receives a complaint about a clinical trial, she says the agency rarely has the staff available to show up and inspect. And sometimes oversight occurs too late….“There’s just a complete lack of oversight of contract research organisations and independent clinical research facilities,” says Jill Fisher, professor of social medicine at the University of North Carolina School of Medicine and author of Medical Research for Hire: The Political Economy of Pharmaceutical Clinical Trials.
“People working in clinical research are terrified of FDA audits,” Jill Fisher told The BMJ, but added that the agency rarely does anything other than inspect paperwork, usually months after a trial has ended. “I don’t know why they’re so afraid of them,” she said. But she said she was surprised that the agency failed to inspect Ventavia after an employee had filed a complaint. “You would think if there’s a specific and credible complaint that they would have to investigate that,” Fisher said.
https://www.bmj.com/content/375/bmj.n2635
Bedevilled by its own internal conflicts of interests and schizophrenia from its competing functions, the FDA has no will, reason or staff to carry out a full audit on Pfizer’s trial in 153 sites strewn across the globe with multiple languages and time zones. It is an impossible undertaking in every possible sense. Throughout the covid pandemic the FDA has abandoned what ever little it may have left of objectivity in favour of suppressing early treatments and vaccine adverse events in order to put all of its efforts into promoting dysfunctional vaccines.
If we cannot audit Pfizer’s data and processes then given the information we have gleaned so far the results of Pfizer’s trial should be thrown out as complete junk most probably of a fraudulent nature. I see no valid reason for Pfizer to be able to continue to hold no legal liability for the damage done by a product that it fraudulently brought to market by faking its apparent efficacy and doing all in its power to conceal the true and very high levels of adverse events and high number of fatalities and serious injuries that have resulted from this. Companies committing fraud should not be given any legal immunity against those seeking compensation for damages caused by their products.
Brilliant synopsis my friend. Please submit this to Robert Malone and Steve Kirsch’s substacks for a feature article.
I wish I could but are not aware of how I would do what you suggest. Many thanks.
This is an extraordinarily useful critique of the problems with the systems for drug review and approval. Many thanks for taking the time to record and share your insights.
Thanks April. Hope you had a good Christmas and wishing you a happy new year.
Excellent article. Thank you for the indepth dive and research. Blessings 🌷